Phase II – Achievements
Phase II – Achievements
The scientific outcome of the second funding period was extraordinarily fruitful surpassing even that of the first funding period. Many major achievements along the proposed research themes have been accomplished in and among all research areas of the SFB 953. Significantly, the level of interdisciplinary collaboration between the PIs spanning the fields of synthesis, physical characterization, device processing, and theory (quantum mechanical calculations) is very high. This, in turn, guaranteed the in-depth investigation of many fundamental, but also more applied topics in the field of synthetic carbon allotropes. Within the last years, the Friedrich-Alexander University of Erlangen-Nürnberg (FAU) was recognized as the leading carbon center worldwide. The funding instrument of the SFB 953 provides the organizational und financial basis for this success.
 We have achieved fundamental breakthroughs with the systematic and comparative exploration of reductive synthesis protocols. For this purpose, we have activated graphene, carbon nanotubes, and fullerenes with alkali metals and studied the resulting intercalation compounds AMnSCA (AM = Li, Na, K) with respect to their physical and chemical properties such as an increased reactivity towards covalent addend binding. For example, we carried out the first in situ Raman monitoring of the reaction of graphite intercalation compounds with small electrophilic molecules exposed as gases. This allowed for the unequivocal assignment and resolution of individual lattice vibrational – Raman – modes of covalently functionalized graphene adducts. The evolution of these bands with increasing degree of functionalization from low to moderate levels provides a basis for the deconvolution of different components towards quantifying the extend of functionalization. By complementary DFT calculations, we were able to identify the vibrational changes in close proximity of the addend-bearing lattice C atoms and assign them to specific Raman modes. A particularly exciting discovery of the second funding period was the quantitative discharging of reduced graphite forms, such as graphite intercalation compounds (GICs), graphenide dispersions, and graphenides deposited on surfaces with benzonitrile applied as a simple liquid. Because of its relatively low reduction potential, benzonitrile is reduced during this process to the radical anion, which exhibits a red color and therefore serves as a reporter molecule for the quantitative determination of the amount of negative charges originally applied on the carbon sheets. Moreover, this discovery reveals a very fundamental physical-chemical phenomenon, namely, a quantitative solvent reduction induced and electrostatically driven mass transport of K+ ions from the solid GIC to the liquid phase. The simple treatment of dispersed graphenides suspended on silica substrates with benzonitrile also leads to a clean conversion to graphene. This unprecedented procedure represents a rather mild, scalable, and inexpensive method for graphene production surpassing previous wet-chemical processes.
We have achieved fundamental breakthroughs with the systematic and comparative exploration of reductive synthesis protocols. For this purpose, we have activated graphene, carbon nanotubes, and fullerenes with alkali metals and studied the resulting intercalation compounds AMnSCA (AM = Li, Na, K) with respect to their physical and chemical properties such as an increased reactivity towards covalent addend binding. For example, we carried out the first in situ Raman monitoring of the reaction of graphite intercalation compounds with small electrophilic molecules exposed as gases. This allowed for the unequivocal assignment and resolution of individual lattice vibrational – Raman – modes of covalently functionalized graphene adducts. The evolution of these bands with increasing degree of functionalization from low to moderate levels provides a basis for the deconvolution of different components towards quantifying the extend of functionalization. By complementary DFT calculations, we were able to identify the vibrational changes in close proximity of the addend-bearing lattice C atoms and assign them to specific Raman modes. A particularly exciting discovery of the second funding period was the quantitative discharging of reduced graphite forms, such as graphite intercalation compounds (GICs), graphenide dispersions, and graphenides deposited on surfaces with benzonitrile applied as a simple liquid. Because of its relatively low reduction potential, benzonitrile is reduced during this process to the radical anion, which exhibits a red color and therefore serves as a reporter molecule for the quantitative determination of the amount of negative charges originally applied on the carbon sheets. Moreover, this discovery reveals a very fundamental physical-chemical phenomenon, namely, a quantitative solvent reduction induced and electrostatically driven mass transport of K+ ions from the solid GIC to the liquid phase. The simple treatment of dispersed graphenides suspended on silica substrates with benzonitrile also leads to a clean conversion to graphene. This unprecedented procedure represents a rather mild, scalable, and inexpensive method for graphene production surpassing previous wet-chemical processes.
 We have pushed forward the general understanding of topochemical principles inherent to covalent graphene functionalization. For the first time, we studied the bis-functionalization of graphene by employing two successive reduction and covalent bond-forming steps. Bulk functionalization in dispersion and functionalization of individual sheets deposited on surfaces was compared. Whereas in the former case attacks from both sides of the plane are possible and can lead to strain-free architectures, in the latter case, retro-functionalizations are seen complementing earlier observations in fullerene and carbon nanotube chemistry. Another fundamental discovery was the experimental and theoretical proof that monolayer graphene is considerably more reactive than bilayer graphene. Supported by DFT calculations, we demonstrated that ditopic addend binding leads to much more stable products than the corresponding monotopic reactions. This is due to the fact that the subsequent monotopic (supratopic) additions to adjacent lattice C atoms lead to highly strained adducts whereas ditopic (antaratopic) additions can lead to completely strain-free situations (e.g., 2D diamond). This is an unprecedented and fundamental insight to graphene chemistry. Moreover, we were able to demonstrated that the preferred addition mode of graphene functionalization proceeds via all-trans hexaadditions to six-membered rings of the graphene lattice-forming perhydro-benzene-holes. These are generated either at rim-defects (graphene edges) or defects in the basal plane. Once a perhydro-benzene-holes is formed, further growth proceeds via perhydro-naphthalene-holes, perhydro-phenanthrene-holes, and so on. We name this process perhydro-PAH-annulations (PAH: polycyclic aromatic hydrocarbons). Two-dimensional disk-like growth is favored over linear growth. As a consequence, continuous growth patterns are generated starting from defects. These patterns contain rims or disk-like islands of per-functionalized graphane (2D diamond, sp3 regions) located next to intact sp2 graphene regions.
We have pushed forward the general understanding of topochemical principles inherent to covalent graphene functionalization. For the first time, we studied the bis-functionalization of graphene by employing two successive reduction and covalent bond-forming steps. Bulk functionalization in dispersion and functionalization of individual sheets deposited on surfaces was compared. Whereas in the former case attacks from both sides of the plane are possible and can lead to strain-free architectures, in the latter case, retro-functionalizations are seen complementing earlier observations in fullerene and carbon nanotube chemistry. Another fundamental discovery was the experimental and theoretical proof that monolayer graphene is considerably more reactive than bilayer graphene. Supported by DFT calculations, we demonstrated that ditopic addend binding leads to much more stable products than the corresponding monotopic reactions. This is due to the fact that the subsequent monotopic (supratopic) additions to adjacent lattice C atoms lead to highly strained adducts whereas ditopic (antaratopic) additions can lead to completely strain-free situations (e.g., 2D diamond). This is an unprecedented and fundamental insight to graphene chemistry. Moreover, we were able to demonstrated that the preferred addition mode of graphene functionalization proceeds via all-trans hexaadditions to six-membered rings of the graphene lattice-forming perhydro-benzene-holes. These are generated either at rim-defects (graphene edges) or defects in the basal plane. Once a perhydro-benzene-holes is formed, further growth proceeds via perhydro-naphthalene-holes, perhydro-phenanthrene-holes, and so on. We name this process perhydro-PAH-annulations (PAH: polycyclic aromatic hydrocarbons). Two-dimensional disk-like growth is favored over linear growth. As a consequence, continuous growth patterns are generated starting from defects. These patterns contain rims or disk-like islands of per-functionalized graphane (2D diamond, sp3 regions) located next to intact sp2 graphene regions.
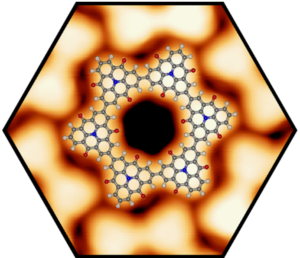 We successfully elaborated the self-assembly and controlled synthesis of zero-dimensional (0D), one-dimensional (1D), and two-dimensional (2D) carbon-rich materials from rationally designed molecular precursors on metal surfaces. We determined their structural and electronic properties at the atomic-scale using high-resolution scanning probe microscopy and spectroscopy. In particular, we demonstrated the defect-free fabrication of covalent porous nanostructures, including macrocycles, nanoribbons, and 2D networks. We studied various approaches to improve the structural control in on-surface synthesis experiments, such as using hierarchical synthesis procedures or self-assemblies as templates. Thereby, we employed Ullmann-type coupling reactions but also explored new surface-catalyzed reactions schemes such as coordination controlled C-C coupling or isomerization reactions in combination with cyclodehydrogenation. For example, we investigated the electronic band gap narrowing from single triphenylamine molecules to covalently-linked triphenylamine chains and 2D networks using a hierarchical on-surface synthesis. In addition, we succeeded to fabricate well-ordered organometallic graphyne-like (sp-sp2) networks, which benefited from the crystallization into large networks upon annealing. Interestingly, these networks showed delocalized unoccupied electronic states, which implicates good charge transport along the layer. We also formed and characterized 2D heterocyclic 2D-networks (heterographene models) by cross-linking of porphyrin precursors in metal surfaces. For the formation of nanographenes on metal-oxide surfaces we have demonstrated that cyclodehydrofluorination of fluorinated precursors is a highly efficient method. These results open promising opportunities towards custom-designed carbon nanostructures by direct on-surface synthesis methods employing technologically relevant insulating surfaces. This breakthrough could provide effective and simple access to innovative carbon-based nano-devices. We have also addressed the challenging topic of interfacial chemical reactions taking place in the confined space between graphene and the underlying substrate. We were able to show that graphene is a monoatomically thin barrier for gases, even up to high pressures of several hundred bar. Following the intercalation of oxygen, we were able to form a NiO layer separating graphene from the metallic nickel surface. The corrugated Moire surface of lattice-mismatched 2D materials was used as template to form well-ordered nanocluster arrays. These serve as model systems for real supported catalysts. Consequently, we obtained detailed insights into their heterogeneous catalytic properties.
We successfully elaborated the self-assembly and controlled synthesis of zero-dimensional (0D), one-dimensional (1D), and two-dimensional (2D) carbon-rich materials from rationally designed molecular precursors on metal surfaces. We determined their structural and electronic properties at the atomic-scale using high-resolution scanning probe microscopy and spectroscopy. In particular, we demonstrated the defect-free fabrication of covalent porous nanostructures, including macrocycles, nanoribbons, and 2D networks. We studied various approaches to improve the structural control in on-surface synthesis experiments, such as using hierarchical synthesis procedures or self-assemblies as templates. Thereby, we employed Ullmann-type coupling reactions but also explored new surface-catalyzed reactions schemes such as coordination controlled C-C coupling or isomerization reactions in combination with cyclodehydrogenation. For example, we investigated the electronic band gap narrowing from single triphenylamine molecules to covalently-linked triphenylamine chains and 2D networks using a hierarchical on-surface synthesis. In addition, we succeeded to fabricate well-ordered organometallic graphyne-like (sp-sp2) networks, which benefited from the crystallization into large networks upon annealing. Interestingly, these networks showed delocalized unoccupied electronic states, which implicates good charge transport along the layer. We also formed and characterized 2D heterocyclic 2D-networks (heterographene models) by cross-linking of porphyrin precursors in metal surfaces. For the formation of nanographenes on metal-oxide surfaces we have demonstrated that cyclodehydrofluorination of fluorinated precursors is a highly efficient method. These results open promising opportunities towards custom-designed carbon nanostructures by direct on-surface synthesis methods employing technologically relevant insulating surfaces. This breakthrough could provide effective and simple access to innovative carbon-based nano-devices. We have also addressed the challenging topic of interfacial chemical reactions taking place in the confined space between graphene and the underlying substrate. We were able to show that graphene is a monoatomically thin barrier for gases, even up to high pressures of several hundred bar. Following the intercalation of oxygen, we were able to form a NiO layer separating graphene from the metallic nickel surface. The corrugated Moire surface of lattice-mismatched 2D materials was used as template to form well-ordered nanocluster arrays. These serve as model systems for real supported catalysts. Consequently, we obtained detailed insights into their heterogeneous catalytic properties.
 We have considerably broadened the field of functional hexa-peri-hexabenzocoronenes (HBCs) via the synthesis and characterization of several families of unprecedented and fascinating architectures. Examples are porphyrin-hexabenzocoronene conjugates as part of our approach towards carbon-rich porphyrins (graphyrines). Up to four HBCs were placed around a porphyrin and up to six porphyrins were connected to one central HBC unit. Crystal structures reveal the HBC dimer formation in the crystals dominating the packing. Furthermore, London dispersion stabilizes porphyrin-HBC clusters in the gas phase. As another exciting HBC-family, we have explored helical structures such helical nanographenes which we denote “superhelicenes”. These systems show massively enhanced fluorescence quantum yields of ~80% compared with ~10% of standard HBCs – and strong solvatochromism due to internal charge transfer processes. Enantiomeric resolution was achieved and allows for entry into novel materials with chiroptical responses, i.e., emission of polarized light, filters, etc. A fundamental factor of overarching relevance to SCA chemistry is the role of defects with respect to covalent addition reactions. To address this question in a systematic manner, we investigated the reductive functionalization of HBC as a model case. The separation and complete characterization of alkylation products obtained from the treatment of HBC2- with alkyl iodides demonstrates that nanographene functionalization proceeds with exceptionally high regio- and stereoselectivities. Experimental and theoretical studies lead to the conclusion that the intact basal graphene plane is chemically inert and addend binding takes place only at preexisting defects or at the periphery (edge defects).
We have considerably broadened the field of functional hexa-peri-hexabenzocoronenes (HBCs) via the synthesis and characterization of several families of unprecedented and fascinating architectures. Examples are porphyrin-hexabenzocoronene conjugates as part of our approach towards carbon-rich porphyrins (graphyrines). Up to four HBCs were placed around a porphyrin and up to six porphyrins were connected to one central HBC unit. Crystal structures reveal the HBC dimer formation in the crystals dominating the packing. Furthermore, London dispersion stabilizes porphyrin-HBC clusters in the gas phase. As another exciting HBC-family, we have explored helical structures such helical nanographenes which we denote “superhelicenes”. These systems show massively enhanced fluorescence quantum yields of ~80% compared with ~10% of standard HBCs – and strong solvatochromism due to internal charge transfer processes. Enantiomeric resolution was achieved and allows for entry into novel materials with chiroptical responses, i.e., emission of polarized light, filters, etc. A fundamental factor of overarching relevance to SCA chemistry is the role of defects with respect to covalent addition reactions. To address this question in a systematic manner, we investigated the reductive functionalization of HBC as a model case. The separation and complete characterization of alkylation products obtained from the treatment of HBC2- with alkyl iodides demonstrates that nanographene functionalization proceeds with exceptionally high regio- and stereoselectivities. Experimental and theoretical studies lead to the conclusion that the intact basal graphene plane is chemically inert and addend binding takes place only at preexisting defects or at the periphery (edge defects).
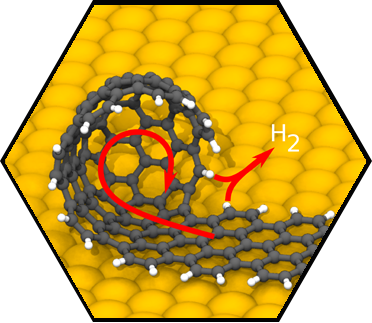 We have accomplished first very encouraging breakthroughs on a central challenge within SCA research, namely, the rational synthesis of atomically precise carbon-based nanostructures including single-walled carbon nanotubes (SWCNTs), isomer-pure fullerenes, buckybowls, nanocones, nanographenes (NGs) and nanoribbons (CNRs). For example, a major achievement was the successful development of a facile strategy to provide SWCNT seeds of virtually any desired chirality. As of the submission of this application, we were able to prepare SWCNT seeds for 21 different helicities including seeds for highly interesting semiconducting tubes. Having the required SWCNT seeds in hand, we were able to demonstrate the first rational synthesis of chirality-pure semiconducting (7,5)-SWCNTs. Moreover, we have demonstrated that our approach can be extended to the synthesis of rationally functionalized nanostructures. In particular, we have demonstrated the general possibility of the controlled fabrication of carbon-based nanostructures with incorporated porphyrin “window”. Finally, we have successfully tackled the long-standing challenge of the bottom-up fabrication of atomically precise carbon-based nanostructures directly on technologically relevant non-metallic substrates.
We have accomplished first very encouraging breakthroughs on a central challenge within SCA research, namely, the rational synthesis of atomically precise carbon-based nanostructures including single-walled carbon nanotubes (SWCNTs), isomer-pure fullerenes, buckybowls, nanocones, nanographenes (NGs) and nanoribbons (CNRs). For example, a major achievement was the successful development of a facile strategy to provide SWCNT seeds of virtually any desired chirality. As of the submission of this application, we were able to prepare SWCNT seeds for 21 different helicities including seeds for highly interesting semiconducting tubes. Having the required SWCNT seeds in hand, we were able to demonstrate the first rational synthesis of chirality-pure semiconducting (7,5)-SWCNTs. Moreover, we have demonstrated that our approach can be extended to the synthesis of rationally functionalized nanostructures. In particular, we have demonstrated the general possibility of the controlled fabrication of carbon-based nanostructures with incorporated porphyrin “window”. Finally, we have successfully tackled the long-standing challenge of the bottom-up fabrication of atomically precise carbon-based nanostructures directly on technologically relevant non-metallic substrates.
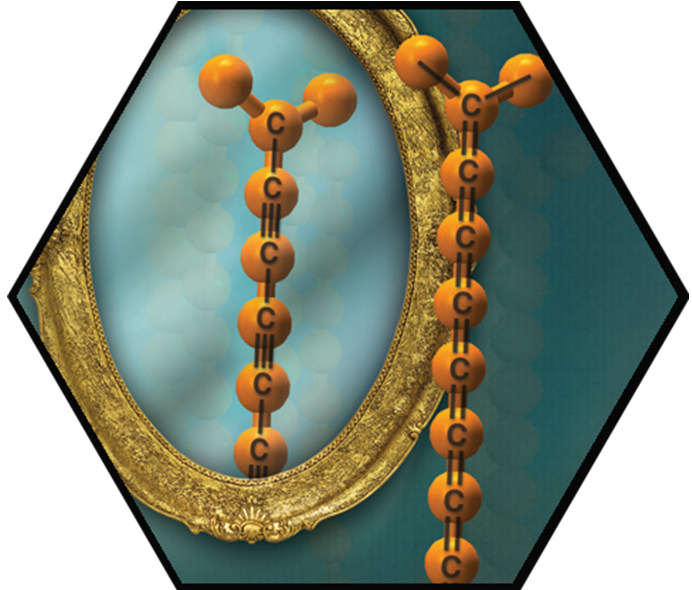 The formation of polyynes and cumulenes approaching carbyne has been successfully explored. Our work with extended cumulenes was particularly innovative and suggested that a combination of steric and electronic considerations is the key to stabilization. In view of the instability shown by all other reported cumulenes without rotaxination, our sp-chain structures show unprecedented stability (persistence). As a solid, C18-TrTol, for example, can be handled for a few hours when kept under N2 atmosphere, while solutions of C18-TrTol in dry de-oxygenated CH>2Cl2 were stable for a few weeks when kept at –20 °C and shielded from ambient light. In view of this stability, the series was extended to the formation of the C22- and C26TrTol derivatives, which could be characterized in solution by UV-Vis absorption. The C22- and C26TrTol cumulenes are the longest known examples to date, to the best of our knowledge. With our extreme carbyne models at hand, we also studied fundamental questions with textbook character such as a rotational barrier around the sp-sp-bonds. Polyynes were also examined as molecular wires. For this purpose, conductance measurement in gold STM break-junctions were carried out.
The formation of polyynes and cumulenes approaching carbyne has been successfully explored. Our work with extended cumulenes was particularly innovative and suggested that a combination of steric and electronic considerations is the key to stabilization. In view of the instability shown by all other reported cumulenes without rotaxination, our sp-chain structures show unprecedented stability (persistence). As a solid, C18-TrTol, for example, can be handled for a few hours when kept under N2 atmosphere, while solutions of C18-TrTol in dry de-oxygenated CH>2Cl2 were stable for a few weeks when kept at –20 °C and shielded from ambient light. In view of this stability, the series was extended to the formation of the C22- and C26TrTol derivatives, which could be characterized in solution by UV-Vis absorption. The C22- and C26TrTol cumulenes are the longest known examples to date, to the best of our knowledge. With our extreme carbyne models at hand, we also studied fundamental questions with textbook character such as a rotational barrier around the sp-sp-bonds. Polyynes were also examined as molecular wires. For this purpose, conductance measurement in gold STM break-junctions were carried out.
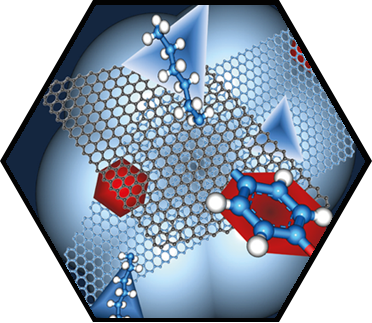
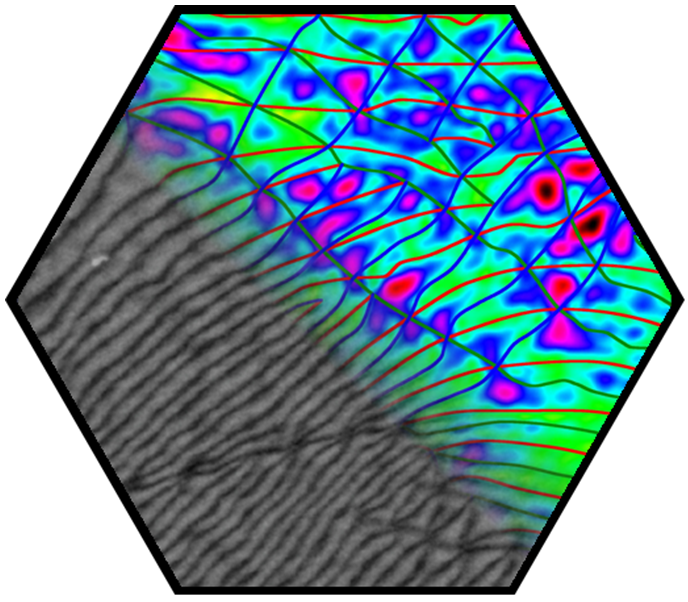 In the first funding period, partial dislocations were observed in bilayer graphene and characterized by transmission electron microscopy, supported by ab initio calculations. These linear topological defects separate areas of different stacking order, resulting in a mosaic structure comprising tiles of AB and BA stacking. These findings stimulated intense research in the second funding period. The presence of partial dislocations drastically changes the electronic structure and electron transport, giving rise to rich phenomena. Theoretical studies emphasized their Janus-headed impact on charge transport, resulting either in perfect conduction or perfect suppression of electronic transparency, depending on geometric details. In a more general approach, the phenomena related to 2D-mosaic materials were studied, resulting for example in fractional conductance plateaus in the quantum regime, and linear magnetoresistivity. The latter effect, an 80-year-old enigma in solid-state physics, is now understood. A further breakthrough was achieved with the direct in situ manipulation of partial dislocations inside an electron microscope. For this, the microscopic investigation of dislocations has been transferred from the transmission electron microscope (TEM) into the scanning electron microscope (SEM), which provides improved contrast due to the use of lower electron energies and higher flexibility in sample manipulation. An in situ mechanical cleaning procedure has been established which uses micro-manipulators inside the SEM to completely remove organic residuals from both sides of the graphene membrane, enabling defect characterization with ultimate quality. Making use of the ultrathin nature of bilayer graphene and the surface topography associated with basal plane dislocations, highly controlled in situ manipulation of individual dislocations has been demonstrated for the first time, which is a major breakthrough in the research on dislocations. The manipulation of dislocations enabled direct testing of fundamental dislocation properties, including line tension, mutual dislocation interaction, and node formation. In particular, a topological switching reaction of partial dislocation could be identified which offers new opportunities for defect engineering in graphene and other 2D materials.
In the first funding period, partial dislocations were observed in bilayer graphene and characterized by transmission electron microscopy, supported by ab initio calculations. These linear topological defects separate areas of different stacking order, resulting in a mosaic structure comprising tiles of AB and BA stacking. These findings stimulated intense research in the second funding period. The presence of partial dislocations drastically changes the electronic structure and electron transport, giving rise to rich phenomena. Theoretical studies emphasized their Janus-headed impact on charge transport, resulting either in perfect conduction or perfect suppression of electronic transparency, depending on geometric details. In a more general approach, the phenomena related to 2D-mosaic materials were studied, resulting for example in fractional conductance plateaus in the quantum regime, and linear magnetoresistivity. The latter effect, an 80-year-old enigma in solid-state physics, is now understood. A further breakthrough was achieved with the direct in situ manipulation of partial dislocations inside an electron microscope. For this, the microscopic investigation of dislocations has been transferred from the transmission electron microscope (TEM) into the scanning electron microscope (SEM), which provides improved contrast due to the use of lower electron energies and higher flexibility in sample manipulation. An in situ mechanical cleaning procedure has been established which uses micro-manipulators inside the SEM to completely remove organic residuals from both sides of the graphene membrane, enabling defect characterization with ultimate quality. Making use of the ultrathin nature of bilayer graphene and the surface topography associated with basal plane dislocations, highly controlled in situ manipulation of individual dislocations has been demonstrated for the first time, which is a major breakthrough in the research on dislocations. The manipulation of dislocations enabled direct testing of fundamental dislocation properties, including line tension, mutual dislocation interaction, and node formation. In particular, a topological switching reaction of partial dislocation could be identified which offers new opportunities for defect engineering in graphene and other 2D materials.
 With laser pulses as short as two optical cycles (~5 fs, 1 fs = 10-15 s), we have demonstrated fully coherent electron matter wave control inside of graphene, representing the first demonstration of strong-field and atto-second physics inside of a conductor, with direct implications for future light-field electronics. We have shown subsequent coherent Landau-Zener transitions, known as Landau-Zener-Stückelberg (LZS) interferometry. Based on this physics, we can provide the fastest turnon time of a current of ~1 fs ever demonstrated. By going from linearly polarized light fields to elliptical, we could demonstrate complex electron trajectory control inside graphene and could show that we can turn on and off LZS physics. We have also shown 300 attosecond-fast charge transfer across a graphene-SiC Schottky junction. Furthermore, we have demonstrated a new way of coating and growing nanometer sharp metal tips with nanodiamonds. With their negative electron affinity, nanodiamond-coated tips are highly promising as high-brightness electron sources, and we have shown already that they are more stable under femtosecond laser irradiation than any other high-brightness electron source. We have also identified various emission channels in the wavelength range of 250-1,930 nm. Finally, based on a carbon nanotube electron beamsplitter, we have shown that femtosecond electron pulses from sharp needle tips are as coherent as the most coherent standard electron beam, namely the one emitted from a cold-field emission tip in DC operation.
With laser pulses as short as two optical cycles (~5 fs, 1 fs = 10-15 s), we have demonstrated fully coherent electron matter wave control inside of graphene, representing the first demonstration of strong-field and atto-second physics inside of a conductor, with direct implications for future light-field electronics. We have shown subsequent coherent Landau-Zener transitions, known as Landau-Zener-Stückelberg (LZS) interferometry. Based on this physics, we can provide the fastest turnon time of a current of ~1 fs ever demonstrated. By going from linearly polarized light fields to elliptical, we could demonstrate complex electron trajectory control inside graphene and could show that we can turn on and off LZS physics. We have also shown 300 attosecond-fast charge transfer across a graphene-SiC Schottky junction. Furthermore, we have demonstrated a new way of coating and growing nanometer sharp metal tips with nanodiamonds. With their negative electron affinity, nanodiamond-coated tips are highly promising as high-brightness electron sources, and we have shown already that they are more stable under femtosecond laser irradiation than any other high-brightness electron source. We have also identified various emission channels in the wavelength range of 250-1,930 nm. Finally, based on a carbon nanotube electron beamsplitter, we have shown that femtosecond electron pulses from sharp needle tips are as coherent as the most coherent standard electron beam, namely the one emitted from a cold-field emission tip in DC operation.
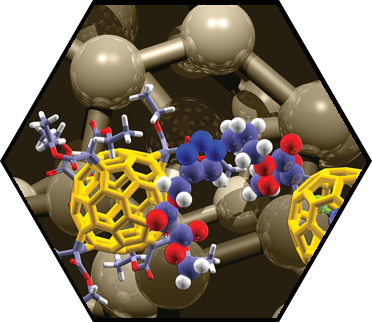
 Several porotypes of unprecedented mechanically interlocked SCA architectures, in which organic macrocycles are “wrapped” around fullerenes or SWCNTs, have been developed. These investigations revealed remarkable insights into the fundamental chemistry of fullerenes, including the realization of rotaxane shuttles with unique charge transfer kinetics between mechanically interlocked building blocks. Further achievements include the generation of modular dyads for photoinduced electron transfer between fullerenes and porphyrins based on a supramolecular [10]CPP junction and the observation of extremely high (108 M-1) fullerene affinity in a porphyrinylene/phenylene nanohoop. Self-assembly of SWCNTs and new nanographene precursors surface engineering (self-assembled monolayers) led to the controlled ordering of SACs with thin film morphology. The studies provided the basis for the design of new thin film and memory transistors. We have also generated highly integrated organic-inorganic architectures consisting of 2D-graphene/graphite, cationic PDI-amphiphiles, and 0D-ZnO nanoparticles (NPs). The full characterization of these hybrid supramolecular architectures revealed the formation of thin films with outstanding centimeter-scale homogeneity, and with an entangled structure exhibiting close contact between the organic and inorganic building blocks.
Several porotypes of unprecedented mechanically interlocked SCA architectures, in which organic macrocycles are “wrapped” around fullerenes or SWCNTs, have been developed. These investigations revealed remarkable insights into the fundamental chemistry of fullerenes, including the realization of rotaxane shuttles with unique charge transfer kinetics between mechanically interlocked building blocks. Further achievements include the generation of modular dyads for photoinduced electron transfer between fullerenes and porphyrins based on a supramolecular [10]CPP junction and the observation of extremely high (108 M-1) fullerene affinity in a porphyrinylene/phenylene nanohoop. Self-assembly of SWCNTs and new nanographene precursors surface engineering (self-assembled monolayers) led to the controlled ordering of SACs with thin film morphology. The studies provided the basis for the design of new thin film and memory transistors. We have also generated highly integrated organic-inorganic architectures consisting of 2D-graphene/graphite, cationic PDI-amphiphiles, and 0D-ZnO nanoparticles (NPs). The full characterization of these hybrid supramolecular architectures revealed the formation of thin films with outstanding centimeter-scale homogeneity, and with an entangled structure exhibiting close contact between the organic and inorganic building blocks.
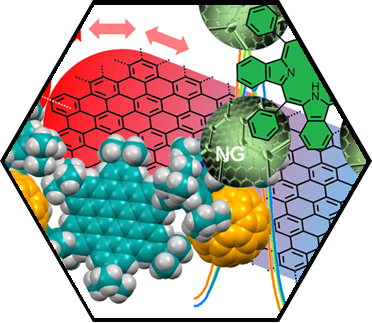
 We have accomplished pioneering contributions towards the development of the first prototypes of inter-SCA-architectures. As an example for inter-fullerene architectures, we have synthesized and characterized the first molecular dumbbell consisting of an endohedral Sc3N@C80 and an empty C60-fullerene building block. The covalent connection of the two fullerenes was accomplished via a simple “click” reaction between the corresponding precursor adducts. Another successful entry into SCA hybrids was realized by modular graphene functionalization with both C60 and the endohedral fullerene Sc3N@C80. These functionalized 2D architectures were synthesized, extending our reductive graphene activation/exfoliation protocols. The detailed characterization by statistical Raman spectroscopy, TG-GC-MS, and LD-TOF mass spectrometry confirmed the covalent bonding of the respective C60/Sc3N@C80 moieties to the basal graphene plane. Furthermore, the formation of the fullerene-graphene hybrids were also visualized by aberration-corrected high-resolution transition electron microscopy HR-TEM. We have also shown that porphyrin-HBC conjugates form inter-carbon allotrope architectures with fullerenes, essentially acting as fullerene sponges.
We have accomplished pioneering contributions towards the development of the first prototypes of inter-SCA-architectures. As an example for inter-fullerene architectures, we have synthesized and characterized the first molecular dumbbell consisting of an endohedral Sc3N@C80 and an empty C60-fullerene building block. The covalent connection of the two fullerenes was accomplished via a simple “click” reaction between the corresponding precursor adducts. Another successful entry into SCA hybrids was realized by modular graphene functionalization with both C60 and the endohedral fullerene Sc3N@C80. These functionalized 2D architectures were synthesized, extending our reductive graphene activation/exfoliation protocols. The detailed characterization by statistical Raman spectroscopy, TG-GC-MS, and LD-TOF mass spectrometry confirmed the covalent bonding of the respective C60/Sc3N@C80 moieties to the basal graphene plane. Furthermore, the formation of the fullerene-graphene hybrids were also visualized by aberration-corrected high-resolution transition electron microscopy HR-TEM. We have also shown that porphyrin-HBC conjugates form inter-carbon allotrope architectures with fullerenes, essentially acting as fullerene sponges.

